By Staffan Lindgren | 5 June 2022

We have heard a fair bit about sponges lately. I presented a “Nature Nugget” on the glass sponge reefs of the Salish Sea and Hecate Strait at the March public meeting, and Tom Hlavac mentioned the glass sponge reefs in his informative presentation at the April meeting. As if on cue, an excellent article by Sheila Byers about the sponge reefs appeared in the spring 2022 of BC Nature Magazine. If you have not read that yet, I strongly recommend that you do so. Because sponge reefs occur at depths of 30+ meters, they are “out of sight, out of mind”. That is unfortunate since they are extremely unique, important, but also highly vulnerable. In Howe Sound, eight reefs have been designated Marine Refuge Conservation Areas, and the remaining five reefs were designated as Glass Sponge Reef Fishery Closures as of January 17 of this year. Unfortunately, a lack of monitoring and enforcement combined with willful defiance of the regulations by both commercial and recreational fishers mean that reefs are still being damaged. Details can be found in the BC Nature article, and you can find additional information at the Marine Life Sanctuaries Society homepage at https://mlssbc.com/.
The glass sponges, Class Hexactinellida, comprise one of four classes of extant sponges (Phylum Porifera). The other Classes are the Calcarea, Demospongiae and the Homoscleromorpha, a recently recognized Class of sponges, with 115 species. Of these, the majority (~7,500 species) are in the Class Demospongiae, which are marine except for a small number of brackish and freshwater species. Demo is Greek for people, and depending on the source, this Class is so named either because they are the most common sponges, or because they tend to be found in groups like people. Approximately 670 species are known in the marine Class Calcarea, and a slightly lower number of species are glass sponges, which are also exclusively marine. Classification is based on the type and morphology of spicules (supportive and defensive structures scattered throughout the sponge body). Spicules are made from aragonite or calcite (types of calcium carbonate) or from silica depending on the Class. Demospongiae can also have a network of spongin fibers, and in the highest quality of sponges harvested commercially (“bath sponges”), spicules are thankfully missing altogether. In the glass sponges spicules may be separate and held together by a soft tissue (a loose skeleton), or they may be fused in a structural network of silica (fused skeleton).

Sponges can have various growth forms, e.g., Globular, Vase, Branching, Amorphous, Encrusting etc., and can vary in size from just a few centimeters to several meters. The ones we see in the intertidal zone around Nanaimo are usually encrusting. With the exception of some predatory species in the Family Cladorhizidae (Class Demospongiae) (Thomassen Hestetun 2017), sponges are filter feeders. The vast majority of them have a complex network of canals and chambers which slows down the water and allows for efficient filtration. They feed on bacteria and other microscopic organisms, although some species feed on dissolved organic matter. Sponges filter water at an astonishing rate and thus are key in maintaining clean water, e.g., the Salish Sea glass sponge reefs filter the entire basin volume of water 3 times per year! They hold promise as a bioremediation agent in aquaculture operations (Zhang et al. 2010), and a recent study found that they also collect DNA from other marine organisms, and may be useful as biological collection vessels for biodiversity monitoring using environmental DNA (eDNA). Sponges also create habitat for other organisms, e.g., the Venus’ flower basket (Euplectella aspergillum ) is one of the most fascinating examples. This beautiful glass sponge is often host to a breeding pair of house glass sponge shrimp in the family Spongicolidae. The shrimp are actually trapped inside the sponge, which in Japan symbolized lasting love. Being sedentary, sponges also defend themselves against predation chemically, which make them interesting as a potential source of pharmaceuticals (Anjum et al. 2016). In addition, sponges are hosts to a large number of microbial symbionts (Webster and Taylor 2011).

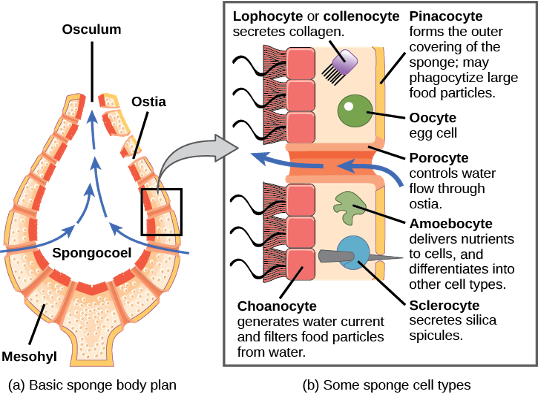
It is the anatomical make-up of sponges that I find of particular interest because sponges represent an apparent evolutionary step between single-celled and multi-celled animals. A recent study did confirm that sponges are indeed the oldest lineage of multicellular animal evolution (Pisani et al. 2015), refuting earlier findings proposing that comb jellies (Phylum Ctenophora) held that honour (although the debate continues, Halanych 2015, Whelan et al. 2017). Similar to all multicellular animals, a sponge consists of a number of cell types. The major ones are pinacocytes, porocytes, choanocytes (or collar cells), sclerocytes, spongocytes and archeocytes (or ameoebocytes). Pinacocytes make up the “skin” of a sponge. Porocytes create pores through which water is drawn into the sponge. Generating the water movement are choanocytes that line the channels, or in the simplest forms the spongocoel, which is the central cavity of a sponge. In Syconoid and Leuconoid body plans, the canal system is increasingly complex). Choanocytes also capture food and reproductive cells. Sclerocytes are amoeboid cells that secrete spicules, and spongocytes secrete the spongin fibers. Archeocytes are amoeba-like cells which move freely in the mesohyl, a jelly-like acellular layer. They collect and digest food and reproductive cells from choanocytes. Most importantly, archeocytes are totipotent, i.e., they can turn into any other type of cell as required. What is most fascinating is that some of these cell types are remarkably similar to Protozoans. For example, archeocytes are essentially like free-living “naked” amoebas (gymnamoebae) and choanocytes are very similar to choanoflagellate protozoans. Some choanoflagellates are colonial, further hinting at a connection between the protozoan and the sponge cell (but see Mah et al. 2014). In glass sponges, the soft tissue is largely made up of a “trabecular reticulum”, which consists of cytoplasm with numerous nuclei not separated by cell membranes (a syncytium). Further details are described in this article.
As a final tidbit of food for thought is the remarkable regenerative powers of sponges. In fact that you can cut up a sponge, force the cells through a sieve, and they will reassemble as a sponge again! Sponges have been on this planet for at least 600 million years. They may have been the first step from unicellular to multicellular organisms and are therefore critical for helping us understand how multicellular life evolved. There is much more to learn about them, however. Check out this video for more!
References
Anjum, K., S.Q. Abbas, S.A. Shah, N. Akhter, S. Batool, and S.S. Hassan. 2016. Marine sponges as a drug treasure. Biomolecules and Therapeutics. 24: 347–362.
Halanych, K.M. 2015. The ctenophore lineage is older than sponges? That cannot be right! Or can it? The Journal of Experimental Biology. 218: 592-597.
Mah, J.L., K.K. Christensen-Dalsgaard, and S.P. Leys. 2014. Choanoflagellate and choanocyte collar-flagellar systems and the assumption of homology. Evolution and Development 16: 25-37.
Pisani, D., W. Pett, M. Dohrmann, R. Feuda, O. Rota-Stabelli, H. Philippe, N. Lartillot, and G. Wörheide. 2015. Genomic data do not support comb jellies as the sister group to all other animals. Proceedings of the National Academy of Sciences, 2015; 201518127
Thomassen Hestetun, J., G. Tompkins-Macdonald, and H.T. Rapp. 2017. A review of carnivorous sponges (Porifera: Cladorhizidae) from the Boreal North Atlantic and Arctic. Zoological Journal of the Linnean Society 181: 1–69.
Webster, N.S., and M.W. Taylor. 2011. Marine sponges and their microbial symbionts: love and other relationships. Environmental Microbiology. 14: 335-346.
Whelan, N.V., K.M. Kocot, T.P. Moroz, K. Mukherjee, P. Williams, G. Paulay, L.L. Moroz, and K.M. Halanych. 2017. Ctenophore relationships and their placement as the sister group to all other animals. Nature Ecology and Evolution. 1: 1737–1746.
Zhang X., W. Zhang, L. Xue, B. Zhang, M. Jin, and W. Fu. 2010. Bioremediation of bacteria pollution using the marine sponge Hymeniacidon perlevis in the intensive mariculture water system of turbot Scophthalmus maximus. Biotechnology and Bioengineering.105: 59-68.
